There is a case of Flyers Fever going around Philly, and we've got it bad here at Casa Spencer. While looking into some schedules and stats and, of course, surfing my education- and teacher-related blogs, I found the NBC Learn site and, more specifically, their site on the Science of Hockey.
Each page features videos (with transcripts!) and detailed lesson plans for teachers to use in classrooms. Being that we are gearing up for our last unit of the year in science, Motion and Forces, and our final foray into Geometry in math, I got pretty excited.
"The Science of NHL Hockey" site includes lessons on Newton's laws of motion, angles, circles and statistics, to name a few. Given that playoff season will be in full swing all month, and hopefully the Flyers will stay alive, I am really looking forward to including these videos and lessons into the last few weeks of school!
Monday, April 30, 2012
The Titanic Fanatic and Ellis Enthusiast
Lately, I was reminded of two topics that were something of an obsession for me when I was younger. Like so many teachers I know, I was something of a nerd, especially for history, art and culture. This was abundantly apparent in the middle grades when popular opinion leaned more toward surfing (in suburban PA? I don't get it either...) and collecting Beanie Babies and pogs.
My elementary teachers used to send home those tissue-thin book order forms from Scholastic, at which point I would immediately begin begging my mom for the Thundercats Sticker Book or Lisa Frank Stationary Kit. On the rare occasion that she would let me get something, it was never of the sticker or colored pencil variety - it was always a book.
From these very book orders, I got two books that fueled a voracious appetite for two specific topics - the Titanic and Ellis Island. The first book was a softcover full-color account of Robert Ballard's discovery of the sunken vessel and I was immediately transfixed. Within months, I had at least ten other books on the subject and my nana and I had watched "A Night to Remember" at least three times. (This was long before the James Cameron film on the subject. I was incredibly excited about the attention to detail in the making of the film, then horribly disappointed by the plot.)
My interest in history and culture was certainly inherited from my mom, so naturally the other book she picked from the catalog was about Ellis Island. Once again, about a year later, a new obsession was forged. Countless books and PBS specials from the library later, we received a flyer for Haverford Township's summer trips series and, lo and behold, there was a bus trip to New York to tour Ellis Island and the Statue of Liberty. In a nutshell, I made my mom ride for three hours on a charter bus despite her motion sickness, then pouted when we had to leave Ellis Island to go to the Statue of Liberty.
My point for rehashing all of these childhood memories is that these passions in our lives as educators can make for some real expertise and enthusiasm in instruction. This year marked the 100th anniversary of the sinking of the Titanic and with these anniversaries come countless opportunities to teach about them. Even though it did not directly fit into the curriculum, the kids had lots of questions about the disaster as a result of the media buzz and rerelease of the 1997 film. I told them about my interest when I was their age and at least a few checked Titanic-themed books out from the library.
When I checked my email this morning, I found that Scholastic Publishers themselves, makers of the book order forms that started it all, have launched a virtual Ellis Island exhibit on their site. They have a virtual tour, as well as tons of images, graphs and infographics about the immigration site. I was excited to see the feature, yet disappointed that there was no foreseeable way for me to fit this into my current classes. So, I just shared it with my colleagues in the social studies department and hoped that it would be of use to them.
Obviously, most of my students will not become avid enthusiasts of any of the topics that inspired me as a youth, but I hope that something we cover this year inspires them to become experts on their own. There is no better way to read and research than to do so about a topic that fascinates you. All I can hope to do is to cover enough diverse subject matter - from rocks to physics and ancient mythology to apartheid - in my three courses that I can light that spark in at least a few kids.
Until then, I will be taking a virtual tour of Ellis Island online and hoping to convince my family to trek back there again this summer...
My elementary teachers used to send home those tissue-thin book order forms from Scholastic, at which point I would immediately begin begging my mom for the Thundercats Sticker Book or Lisa Frank Stationary Kit. On the rare occasion that she would let me get something, it was never of the sticker or colored pencil variety - it was always a book.
From these very book orders, I got two books that fueled a voracious appetite for two specific topics - the Titanic and Ellis Island. The first book was a softcover full-color account of Robert Ballard's discovery of the sunken vessel and I was immediately transfixed. Within months, I had at least ten other books on the subject and my nana and I had watched "A Night to Remember" at least three times. (This was long before the James Cameron film on the subject. I was incredibly excited about the attention to detail in the making of the film, then horribly disappointed by the plot.)
My interest in history and culture was certainly inherited from my mom, so naturally the other book she picked from the catalog was about Ellis Island. Once again, about a year later, a new obsession was forged. Countless books and PBS specials from the library later, we received a flyer for Haverford Township's summer trips series and, lo and behold, there was a bus trip to New York to tour Ellis Island and the Statue of Liberty. In a nutshell, I made my mom ride for three hours on a charter bus despite her motion sickness, then pouted when we had to leave Ellis Island to go to the Statue of Liberty.
My point for rehashing all of these childhood memories is that these passions in our lives as educators can make for some real expertise and enthusiasm in instruction. This year marked the 100th anniversary of the sinking of the Titanic and with these anniversaries come countless opportunities to teach about them. Even though it did not directly fit into the curriculum, the kids had lots of questions about the disaster as a result of the media buzz and rerelease of the 1997 film. I told them about my interest when I was their age and at least a few checked Titanic-themed books out from the library.
When I checked my email this morning, I found that Scholastic Publishers themselves, makers of the book order forms that started it all, have launched a virtual Ellis Island exhibit on their site. They have a virtual tour, as well as tons of images, graphs and infographics about the immigration site. I was excited to see the feature, yet disappointed that there was no foreseeable way for me to fit this into my current classes. So, I just shared it with my colleagues in the social studies department and hoped that it would be of use to them.
Obviously, most of my students will not become avid enthusiasts of any of the topics that inspired me as a youth, but I hope that something we cover this year inspires them to become experts on their own. There is no better way to read and research than to do so about a topic that fascinates you. All I can hope to do is to cover enough diverse subject matter - from rocks to physics and ancient mythology to apartheid - in my three courses that I can light that spark in at least a few kids.
Until then, I will be taking a virtual tour of Ellis Island online and hoping to convince my family to trek back there again this summer...
Wednesday, April 25, 2012
The Flipped Classroom Experiment Begins!
All year, I have been experimenting with the Camtasia software to record videos of my laptop screen. Most of my videos, however, were designed to demonstrate some important function, like signing on to our school's VPN or using the WYNN text-to-speech program. I have yet to try a truly instructional video with my students.
As we approach the end of the year and the pages in my plan book are actually labeled all the way through exams in June, I realized that we are in "crunch time." We are behind where we were last year, but mostly because we added a unit of study back in October that the kids really wanted. Either way, we have an entire unit to fit in before exams in June and I am really getting creative on how to cram it all in.
A few days ago, I decided to create a flipped classroom video of one of the sections of the textbook and the notes that go along with it. I planned on assigning the 18-minute video over this long weekend so that we can fit another lab in before the quiz instead of using all of our classtime for reading and notetaking. It is also almost time to begin my presentation on my Personalized Professional Development concept - flipped classroom - and I need something to present!
I learned a few things while preparing this video today. First of all, for every minute of video, plan to spend about 3 minutes getting it ready. It took me about 5 minutes to set up my laptop for recording, 18 minutes to record it, and another 20 minutes to edit. Once it was ready, the computer must "render" and "produce" the mp4 file. I have no idea what the computer is doing while it is rendering, but I do know it takes about as many minutes as the video is long. I went and made some copies while it did its thing.
Uploading the video to my SchoolTube Channel only took a few moments, then finally my video was ready for students to view. As I looked up at the clock this afternoon and realized that it took about an hour to make my 20-minute work of art, I briefly wondered if it was all worth it.
It should be fairly obvious that the best days in science class are those packed with labs and activities. We often get into great discussions on reading notes days, but they're not really any one's favorite. On a typical reading notes day, I would spend most of the time walking around the room listening to the kids read the text, then filling in with explanations. The classes are 45 minutes each and I repeat this same process three times back-to-back.
Even though the creation of the video took about an hour, I am saving myself and the students a little over two hours of traditional instruction. Theoretically, the students can also go through the video at their own pacing. If a student needs to spend more time, they can, and they need not be whisked along at the speed of the rest of us. There are also precious few days of the rocks unit left and I would rather fill them with labs and activities than more reading and notes. In the end, I decided that it was definitely worth it.
Tomorrow I will give the kids a quick run-through of how to access the video. Given that SchoolTube operates almost exactly like YouTube, the kids should be pros right away. I will also explain my expectations for the notes. When the kids return to school on Monday, I would like to conduct a quick survey about how the experience was for them.
I certainly hope that it is a success, but either way, I will post the results here!
As we approach the end of the year and the pages in my plan book are actually labeled all the way through exams in June, I realized that we are in "crunch time." We are behind where we were last year, but mostly because we added a unit of study back in October that the kids really wanted. Either way, we have an entire unit to fit in before exams in June and I am really getting creative on how to cram it all in.
A few days ago, I decided to create a flipped classroom video of one of the sections of the textbook and the notes that go along with it. I planned on assigning the 18-minute video over this long weekend so that we can fit another lab in before the quiz instead of using all of our classtime for reading and notetaking. It is also almost time to begin my presentation on my Personalized Professional Development concept - flipped classroom - and I need something to present!
I learned a few things while preparing this video today. First of all, for every minute of video, plan to spend about 3 minutes getting it ready. It took me about 5 minutes to set up my laptop for recording, 18 minutes to record it, and another 20 minutes to edit. Once it was ready, the computer must "render" and "produce" the mp4 file. I have no idea what the computer is doing while it is rendering, but I do know it takes about as many minutes as the video is long. I went and made some copies while it did its thing.
Uploading the video to my SchoolTube Channel only took a few moments, then finally my video was ready for students to view. As I looked up at the clock this afternoon and realized that it took about an hour to make my 20-minute work of art, I briefly wondered if it was all worth it.
It should be fairly obvious that the best days in science class are those packed with labs and activities. We often get into great discussions on reading notes days, but they're not really any one's favorite. On a typical reading notes day, I would spend most of the time walking around the room listening to the kids read the text, then filling in with explanations. The classes are 45 minutes each and I repeat this same process three times back-to-back.
Even though the creation of the video took about an hour, I am saving myself and the students a little over two hours of traditional instruction. Theoretically, the students can also go through the video at their own pacing. If a student needs to spend more time, they can, and they need not be whisked along at the speed of the rest of us. There are also precious few days of the rocks unit left and I would rather fill them with labs and activities than more reading and notes. In the end, I decided that it was definitely worth it.
Tomorrow I will give the kids a quick run-through of how to access the video. Given that SchoolTube operates almost exactly like YouTube, the kids should be pros right away. I will also explain my expectations for the notes. When the kids return to school on Monday, I would like to conduct a quick survey about how the experience was for them.
I certainly hope that it is a success, but either way, I will post the results here!
Friday, April 20, 2012
The (In)Famous Edible Rocks Lab
It's messy. It's sticky. It's sugary. It's also one of the most memorable and fun labs that we do all year in science. In order to model the way the three different types of rock form, we take a day to make three treats, then describe how each treat is related to either igneous, metamorphic or sedimentary rock.
Each student receives a lab packet that carefully prescribes how to complete each station of the lab and also guides them to making observations about their models. The classroom is divided into three stations, two of which should be manned by a teacher. Luckily, this year we have a student teacher working with us in addition to the pair of us, so we had a teacher at each station.
At the jelly sandwich station, we make simple jelly sandwiches (we have a few peanut allergies in the class so we skipped the PB) then record our observations about the color, appearance and texture of the bread and the jelly. Then, the sandwiches get smushed! We place the sandwiches in a plastic baggie and pile a few textbooks on top. After a few moments, we look back at the sandwiches and record further information. Both the jelly and the bread have changed their texture and appearance drastically. These sandwiches are intended to model the drastic change caused by heat and pressure in metamorphic rocks.
At the chocolate patties station, a teacher assists the kids in melting molding chocolate in the microwave. We chose to mix colors to make a marbled effect, but this is optional. Each student gets to spoon a bit of the mixture into a small Dixie cup. Then they are allowed to cool. While they cool, students record observations in their notes. The chocolate patties represent igneous rocks in that they are made of a molten, melted material that cools over time.
Finally, a teacher is also ready and waiting at the crisp rice treats station. The easiest way to make the treats here at school is with a microwave. Melt about a teaspoon pat of butter in a large bowl, then add about 8 or 9 regular-sized marshmallows. Mix them around until they are coated with butter. Microwave the marshmallows for about a minute until they are warm and "puffed up." Then add about a cup or cup and a half of cereal (I always eyeball it) and let the students mix up the "sediments." We also added some mini M&Ms just to make the sediments a little more interesting. Finally, another student can press the mixture into a large bar pan with a greased spatula. I usually divide my class into three groups, each group filling the pan about a third. In the end, there is a full pan of treats. These crisp rice bars represent sedimentary rocks in that the smaller "sediments" were bonded together through compaction and cementation.
Once the students have visited all three stations, they are ready to answer the reflective questions in the lab packet about which treat represented each type of rock. Usually, by this time, the crisp rice treats are cooled enough to cut up and distribute. Most students do not want to each the jelly sandwiches, and I typically send the chocolate patties home. (We don't need that much sugar right after lunch!)
Many of the 7th, 8th and 9th graders who have moved on from my class still remember this lab fondly, and it is certainly one of the favorites of current 6th graders. We also have teachers come from all over the building to have a sweet treat in the afternoon with our students. While there is certainly some scientific material to be learned from this lab, the best part is seeing students get excited about science!
Each student receives a lab packet that carefully prescribes how to complete each station of the lab and also guides them to making observations about their models. The classroom is divided into three stations, two of which should be manned by a teacher. Luckily, this year we have a student teacher working with us in addition to the pair of us, so we had a teacher at each station.
At the jelly sandwich station, we make simple jelly sandwiches (we have a few peanut allergies in the class so we skipped the PB) then record our observations about the color, appearance and texture of the bread and the jelly. Then, the sandwiches get smushed! We place the sandwiches in a plastic baggie and pile a few textbooks on top. After a few moments, we look back at the sandwiches and record further information. Both the jelly and the bread have changed their texture and appearance drastically. These sandwiches are intended to model the drastic change caused by heat and pressure in metamorphic rocks.
At the chocolate patties station, a teacher assists the kids in melting molding chocolate in the microwave. We chose to mix colors to make a marbled effect, but this is optional. Each student gets to spoon a bit of the mixture into a small Dixie cup. Then they are allowed to cool. While they cool, students record observations in their notes. The chocolate patties represent igneous rocks in that they are made of a molten, melted material that cools over time.
Finally, a teacher is also ready and waiting at the crisp rice treats station. The easiest way to make the treats here at school is with a microwave. Melt about a teaspoon pat of butter in a large bowl, then add about 8 or 9 regular-sized marshmallows. Mix them around until they are coated with butter. Microwave the marshmallows for about a minute until they are warm and "puffed up." Then add about a cup or cup and a half of cereal (I always eyeball it) and let the students mix up the "sediments." We also added some mini M&Ms just to make the sediments a little more interesting. Finally, another student can press the mixture into a large bar pan with a greased spatula. I usually divide my class into three groups, each group filling the pan about a third. In the end, there is a full pan of treats. These crisp rice bars represent sedimentary rocks in that the smaller "sediments" were bonded together through compaction and cementation.
Once the students have visited all three stations, they are ready to answer the reflective questions in the lab packet about which treat represented each type of rock. Usually, by this time, the crisp rice treats are cooled enough to cut up and distribute. Most students do not want to each the jelly sandwiches, and I typically send the chocolate patties home. (We don't need that much sugar right after lunch!)
Many of the 7th, 8th and 9th graders who have moved on from my class still remember this lab fondly, and it is certainly one of the favorites of current 6th graders. We also have teachers come from all over the building to have a sweet treat in the afternoon with our students. While there is certainly some scientific material to be learned from this lab, the best part is seeing students get excited about science!
Flocabulary
Strange name, unusual concept, effectively sticky songs!
Flocabulary is a website, similar to BrainPop!, that features videos of basic content and concepts performed as hip-hop songs. They're a little cheesy, but, boy are they catchy!
Today in class, we watched a video about the elements of short stories, embedded below. The song features famous stories like Jack London's To Build a Fire and discusses the plot, theme, character, setting and conflict in short stories. The songs also feature a repeated refrain that I am still hearing kids humming in the hallway. Given that we are in the midst of a unit on short stories, this fit perfectly into our class.
Just to prove how catchy these refrains are, when asked what the five story elements were after watching the video, each student said them, often unintentionally, in the same order as they were listed in the song, and usually with the same rhythm! Obviously, the song was still swirling in their heads.
Flocabulary is a website, similar to BrainPop!, that features videos of basic content and concepts performed as hip-hop songs. They're a little cheesy, but, boy are they catchy!
Today in class, we watched a video about the elements of short stories, embedded below. The song features famous stories like Jack London's To Build a Fire and discusses the plot, theme, character, setting and conflict in short stories. The songs also feature a repeated refrain that I am still hearing kids humming in the hallway. Given that we are in the midst of a unit on short stories, this fit perfectly into our class.
Just to prove how catchy these refrains are, when asked what the five story elements were after watching the video, each student said them, often unintentionally, in the same order as they were listed in the song, and usually with the same rhythm! Obviously, the song was still swirling in their heads.
Flocabulary is, for the most part, a paid subscription website, though it is very reasonably priced. The site has hundreds of free videos, however, and all of the videos come with accompanying lesson plans and lyric sheets. Each of videos I have watched so far have been among the free selections.
While surfing the site, I came across a video about the area of a triangle, a concept that is fast-approaching in our math unit. Who knows, maybe next week the kids will be singing "area is one half base times height" in the halls instead of the five story elements...
Click here to check out the videos on Flocabulary.
Friday, April 13, 2012
Math Cafe
While studying decimals and percentages, I thought it would be fun to demonstrate one of the everyday uses of these concepts - prices, discounts and tips. To model these concepts, we decided to transform our math class into a restaurant for the day.
Just to add an air of authenticity, I brought in some pads of guest checks for the students to take orders when it was their turn to act as a server. Each student also brought in various snacks to offer in our restaurant. As soon as each student handed me their snack earlier in the morning, I typed up a quick menu of the items we had to offer. Other than that, the preparation was pretty minimal.
Each student took turns as either a server or a customer. We went through the directions and procedure first, then each server got up and took the orders of their customer. They had to record the price of each item, then prepare a plate for the customer. While the customers ate their snacks, the servers added up the totals and, in some cases, applied percentage discounts (like a teacher discount!) and tax. Once a total was reached, the servers dropped the checks and the customers had to determine a tip, then write a check for the total. When all of the steps are complete, the servers may then become customers and the customers became servers.
With any extra time, students also brought menus to other teachers and school employees and this was probably their favorite part of the experience. During the lesson, several students brought up connections that they had heard just from going out with family and friends. It seems to have really made some things "click" from their real-life experience!
Wednesday, April 11, 2012
Mystery Minerals
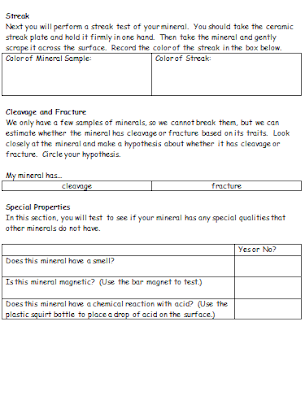
During our minerals unit, we read a section about the physical properties of minerals. The hardness, streak, luster, specific gravity, cleavage, fracture and special properties of a mineral can be used to distinguish it from others that may have a similar appearance. As a lab to complement this lesson, students performed several tests on four "mystery mineral" samples, then had to make hypotheses about the identity of each.
To prepare for the assignment, I created mineral identification kits that included a magnifying glass, a streak plate, a piece of copper, an iron nail, a magnet, and a piece of glass. Because I grouped each class into four groups, I chose four "mystery minerals" to rotate around the room. I chose to include gypsum (for its unusual softness), sulfur (for its unusual appearance), sphalerite (for its unusual reaction to hydrochloric acid), and chalcopyrite (for its sparkly, metallic luster). Later on, we included some samples of calcite just for fun.
Students received a lab packet that walked them through the tests and observations that needed to be recorded. Each group made observations and sketches about the appearance, luster and texture of the minerals. They also performed a streak test on the mineral with the ceramic streak plate, as well as a hardness test with fingernails, pieces of copper, iron nails, pieces of glass and ceramic streak plates. A final section of the packet required that students look for other unique properties, such as magnetism, residue/greasiness, and chemical reactions with acid.
For obvious safety reasons, we performed the acid tests as a whole group with adults applying the hydrochloric acid to the samples. For sulfur, gypsum and chalcopyrite, there is no real reaction or effect when the acid is applied. Sphalerite, however, creates a very noticeable rotten egg odor when hydrochloric acid is applied and it will eventually dissolve if enough acid is used. At this point, we added samples of calcite to the mix because they actually fizz with just a few drops of acid.
At the end of the testing phase, I created a chart, shown below, with the officially recognized geologists' data for each mineral. Students then compared their results to the results in the chart and tried to match each sample to its true identity.
I'm not quite sure why, but this is always one of my favorite labs of the year. We may do many active lessons in this class, but this one really feels like "real science" to the kids because they are using real scientific tools and materials to find real scientific data. At this level of science, most of the so-called "labs" that we complete are really just demonstrations or models, but in this case, it just feels more inquiry-based. Best of all, it can be completed with just a few common objects and a box of mineral samples from your typical school supply catalog!
To prepare for the assignment, I created mineral identification kits that included a magnifying glass, a streak plate, a piece of copper, an iron nail, a magnet, and a piece of glass. Because I grouped each class into four groups, I chose four "mystery minerals" to rotate around the room. I chose to include gypsum (for its unusual softness), sulfur (for its unusual appearance), sphalerite (for its unusual reaction to hydrochloric acid), and chalcopyrite (for its sparkly, metallic luster). Later on, we included some samples of calcite just for fun.
Students received a lab packet that walked them through the tests and observations that needed to be recorded. Each group made observations and sketches about the appearance, luster and texture of the minerals. They also performed a streak test on the mineral with the ceramic streak plate, as well as a hardness test with fingernails, pieces of copper, iron nails, pieces of glass and ceramic streak plates. A final section of the packet required that students look for other unique properties, such as magnetism, residue/greasiness, and chemical reactions with acid.
For obvious safety reasons, we performed the acid tests as a whole group with adults applying the hydrochloric acid to the samples. For sulfur, gypsum and chalcopyrite, there is no real reaction or effect when the acid is applied. Sphalerite, however, creates a very noticeable rotten egg odor when hydrochloric acid is applied and it will eventually dissolve if enough acid is used. At this point, we added samples of calcite to the mix because they actually fizz with just a few drops of acid.
At the end of the testing phase, I created a chart, shown below, with the officially recognized geologists' data for each mineral. Students then compared their results to the results in the chart and tried to match each sample to its true identity.
I'm not quite sure why, but this is always one of my favorite labs of the year. We may do many active lessons in this class, but this one really feels like "real science" to the kids because they are using real scientific tools and materials to find real scientific data. At this level of science, most of the so-called "labs" that we complete are really just demonstrations or models, but in this case, it just feels more inquiry-based. Best of all, it can be completed with just a few common objects and a box of mineral samples from your typical school supply catalog!
Tuesday, April 10, 2012
How many points is your name worth?
Today in math, we took a break from our usual routine to complete an activity related to our most recent unit on lines and angles. The basic idea was to measure the angles in the letters of a name and to add them up to find a point value. At first, we made predictions about whose name would have the most points, but many of results were quite surprising.
To prepare for the activity, I created simple posters of each student's name on 11" x 17" paper. I used the most angular, sans serif font that I could find - Century Gothic. I also created an example with my own name to demonstrate the concept to students.
Each time a student found an angle in his or her name, they marked it and measured it with a protractor, then labeled them. At first, students found just the obvious angles in Ms and As, but as the scores got higher and higher, students searched for more angles to increase their scores and remain competitive.
Overall, the activity was perfect for a 45 minute class period and was a nice wrap-up to our unit on Lines and Angles before the study guide review and quiz. Who knows, maybe next time, we can expand it even further to include identification of types of lines...
To prepare for the activity, I created simple posters of each student's name on 11" x 17" paper. I used the most angular, sans serif font that I could find - Century Gothic. I also created an example with my own name to demonstrate the concept to students.
Each time a student found an angle in his or her name, they marked it and measured it with a protractor, then labeled them. At first, students found just the obvious angles in Ms and As, but as the scores got higher and higher, students searched for more angles to increase their scores and remain competitive.
Overall, the activity was perfect for a 45 minute class period and was a nice wrap-up to our unit on Lines and Angles before the study guide review and quiz. Who knows, maybe next time, we can expand it even further to include identification of types of lines...
Monday, April 2, 2012
Modeling Mitosis
Mitosis is a confusing process if you learn about it without context. When you think about it critically, however, each step has a clear purpose. In order to improve our understanding of mitosis and how it occurs, we decided to model it live in class.
Each student was assigned a role as either cell membrane, centriole, chromosome, or nucleus. In our class, we have 10-11 students, so we selected one nucleus, four students to help with the cell membrane, three students for chromosomes and two centrioles. These numbers can be adjusted as needed, or several cells can be modeled.
At first, we set up a "cell" on the floor with jumprope acting as cell membrane, a large laminated paper disc as the nucleus, laminated pictures of centroles, and chromosomes. The chromosomes were also made of laminated paper, but I created them so that they velcro together on the centromere and can be pulled apart during a later phase.
(I made several colors of the model below. I cut them out, then put velcro dots on the centromere so that they can connect together, then tear apart when necessary.)
Once students were sorted with their roles and had their handouts showing the phases of mitosis, we began our simulation. First, the genetic material in the nucleus bundled together into chromosomes. The centrioles then move to the far sides of the cell and begin to form spindle fibers (modeled by yarn) that extend towards the nucleus. As the nucleus dissolves, the chromosomes line up in a row in the middle of the cell. Each spindle fiber grabs onto one half of a chromosome, then pulls the halves of the chromosomes over to the sides of the cell. At this point, two nuclei form in either side of the cell and the cell membrane students begin pinching in and creating two separate cells.
We had to practice this a few times in order to get it right, but after a few tries, we were able to go through the whole process with little direction from teachers. Overall, it was a fun way to get up out our seats and model a process in action!
Each student was assigned a role as either cell membrane, centriole, chromosome, or nucleus. In our class, we have 10-11 students, so we selected one nucleus, four students to help with the cell membrane, three students for chromosomes and two centrioles. These numbers can be adjusted as needed, or several cells can be modeled.
At first, we set up a "cell" on the floor with jumprope acting as cell membrane, a large laminated paper disc as the nucleus, laminated pictures of centroles, and chromosomes. The chromosomes were also made of laminated paper, but I created them so that they velcro together on the centromere and can be pulled apart during a later phase.
(I made several colors of the model below. I cut them out, then put velcro dots on the centromere so that they can connect together, then tear apart when necessary.)
Once students were sorted with their roles and had their handouts showing the phases of mitosis, we began our simulation. First, the genetic material in the nucleus bundled together into chromosomes. The centrioles then move to the far sides of the cell and begin to form spindle fibers (modeled by yarn) that extend towards the nucleus. As the nucleus dissolves, the chromosomes line up in a row in the middle of the cell. Each spindle fiber grabs onto one half of a chromosome, then pulls the halves of the chromosomes over to the sides of the cell. At this point, two nuclei form in either side of the cell and the cell membrane students begin pinching in and creating two separate cells.
We had to practice this a few times in order to get it right, but after a few tries, we were able to go through the whole process with little direction from teachers. Overall, it was a fun way to get up out our seats and model a process in action!
Growing Crystals
In our book, it describes two ways that crystals can form from solution - when water evaporates and leaves the mineral ions behind, or when the solution is so concentrated with dissolved ions that they form in the solution. We decided to model these two phenomena in our classroom in the simplest way - by showing salt and sugar crystals.
Salt crystals are incredibly easy to show from solution and they look pretty striking when they are done. For our demonstration, we mixed a solution of salt and warm water, then simply poured it into a shallow pan. As the water evaporates away overnight, the slow process allows the salt ions time to re-connect into cubic crystals.
Depending on several variables in the solution, the crystals can be tiny cubic growths, or much larger. Below are some images of both smaller and larger crystals.
Demonstrating crystals forming in solution is a little trickier. We decided to make rock candy in the classroom. In a pot or saucepan, mix two parts sugar to one part water and bring the solution to a boil. This is the only way to ensure that the sugar is completely dissolved. We used a simple lab burner to do so. Once the solution has boiled, you can suspend either a string with a weight on the end or a popsicle stick into the solution. We created a cross-shaped apparatus out of two popsicle sticks and a rubber band to make sure that the stick did not touch the bottom or get stuck to the sides.
The salt crystals are typically ready overnight depending on the depth of the water. I will usually move onward with whatever lesson is scheduled for that day, then allow the students a few minutes at the end of class to analyze their salt crystals and record some data into their lab packets.
The sugar crystals typically take 4-7 days, but some small crystals can be seen in those first few days. Students will also do the regularly-scheduled lesson for that day, then record their observations on the last day of class. Technically, these sugar crystals are edible after a quick rinse and a few minutes to dry.
Often, the sugar crystals can be a bit tricky and tend to form along the sides of the cup or beaker more so than along the stick or string. Either way, crystals will form and they are typically large enough for the kids to pull them out and examine them. Below is an image of sugar crystals that formed predominantly along the inside of the cup.
Salt crystals are incredibly easy to show from solution and they look pretty striking when they are done. For our demonstration, we mixed a solution of salt and warm water, then simply poured it into a shallow pan. As the water evaporates away overnight, the slow process allows the salt ions time to re-connect into cubic crystals.
Depending on several variables in the solution, the crystals can be tiny cubic growths, or much larger. Below are some images of both smaller and larger crystals.
Demonstrating crystals forming in solution is a little trickier. We decided to make rock candy in the classroom. In a pot or saucepan, mix two parts sugar to one part water and bring the solution to a boil. This is the only way to ensure that the sugar is completely dissolved. We used a simple lab burner to do so. Once the solution has boiled, you can suspend either a string with a weight on the end or a popsicle stick into the solution. We created a cross-shaped apparatus out of two popsicle sticks and a rubber band to make sure that the stick did not touch the bottom or get stuck to the sides.
The salt crystals are typically ready overnight depending on the depth of the water. I will usually move onward with whatever lesson is scheduled for that day, then allow the students a few minutes at the end of class to analyze their salt crystals and record some data into their lab packets.
The sugar crystals typically take 4-7 days, but some small crystals can be seen in those first few days. Students will also do the regularly-scheduled lesson for that day, then record their observations on the last day of class. Technically, these sugar crystals are edible after a quick rinse and a few minutes to dry.
Often, the sugar crystals can be a bit tricky and tend to form along the sides of the cup or beaker more so than along the stick or string. Either way, crystals will form and they are typically large enough for the kids to pull them out and examine them. Below is an image of sugar crystals that formed predominantly along the inside of the cup.
Subscribe to:
Posts (Atom)